A miracle therapeutic for some of the most scary diseases
From Cloned Bovines Birthed in South Dakota and Iowa
What if there were a new type of pharmaceutical product that could do things that have never been done before in treating disease? Things like:
· Delaying the onset or progression of Type 1 diabetes.
· Significantly reducing the annual death rate from influenza among older people, who are at high risk because of age and comorbidities.
· Creating a cure for Clostridoides difficile, known as C. diff., an often-fatal disease that tends to arise among hospital patients and nursing home residents, and for which there is no cure.
· Extending the life of victims of the most common type of lung cancer, specifically, non-small-cell lung cancer?
· Creating a cure for COVID-19 that does not become obsolete over time as the disease mutates, a problem lurking just around the corner with the therapeutics that have been developed so far.
All of this and more is happening in the laboratories and offices at SAb Biotherapeutics, Inc., a small, mostly unheard-of company located in rented space in Sioux Falls, South Dakota, and with historical ties to Sioux Center, Iowa.
How I Became Involved With All of This
This column tells the remarkable story of a breakthrough scientific discovery and an entirely new class of therapeutics that is being created. Everything in this column is taken from a book I recently wrote: The Quarter-Quarter-Century Search for a Miracle Therapeutic: The Story of SAb Biotherapeutics and its predecessor companies with a brief history of therapeutic medicine. The book was recently published privately by two of the principals in the company, who hired me to write it. I am also an investor in the company.
Which means, of course, that I have a vested interest in the company’s success. But I was being paid for my time regardless what happens with these new miracle therapeutics. Also, I have been a journalist all my adult life, and I held my work on this story to the same standard I always have used. Tell the truth and let the chips fall where they may. The company has had numerous ups and downs over the years, and they are all chronicled in the book. This column, however, focuses on the potential for these new drugs for the future.
It could be one of the most dramatic developments in therapeutic medicine of the past 135 years, since the development of the world’s first antibody, which provided a cure for one of the world’s most feared killers, diphtheria.
A New Kind of Science, A New Class of Drugs
Remember Dolly the sheep? Born in Scotland in 1996, she was the world’s first mammal cloned from a cell. A little more than a year later, the same cloning process was used by two University of Massachusetts, Amherst scientists to create three cloned bovines.
James Robl, one of the lead researchers on this project, decided that the most promising use for this new cloning technology would be to create cloned bovines that would be genetically engineered to have fully human immune systems.
Robl joined with two fellow scientists in Amherst and a lawyer-businessman from Connecticut in starting a new company, Hematech, in 1998 to try to make this happen. In 2002, Hematech moved to Sioux Falls, South Dakota to get closer to cattle country. In 2012, it became a part of Sanford Health Care in Sioux Falls as Sanford Applied Biosciences, and in 2014, it became an independent company known as SAb Biotherapeutics.
The work initially undertaken by Hematech was an enormous scientific challenge that took years to perfect. It became one of the most complex bodies of scientific work that ever has been undertaken, surpassing both the Manhattan Project and the mapping of the human genome, Robl said.
A fully human immune system had to be genetically created in a laboratory and inserted into a bovine embryo that could then be transferred into a cow through the cloning process. The resulting cloned bovine then could be injected with an antigen for a particular human disease, and its fully human immune system would begin developing fully human antibodies for that disease. The antibodies then could be extracted from the cow via plasma collection, purified, standardized, and made into therapeutics for the specific disease.
These antibodies are different, and more effective than any other man-made antibodies. They are polyclonal antibodies, meaning that they grasp antigens in the body at multiple points. Polyclonal antibodies are what the human body itself manufactures to fight diseases that invade the body. By contrast, the most advanced therapeutics prior to these fully human antibodies have been monoclonal, meaning that they grasp the antigens at just one point. They work, but they are limited in what they can do in fighting many diseases, and there are many diseases for which no monoclonals have yet been developed. In addition, unlike monoclonal therapeutics, these polyclonals are fully human and do not lose their effectiveness over time, or produce side effects that make the people who use them ill.
Until the recent creation of polyclonal antibodies by SAb Biotherapeutics, monoclonals were the last entirely new class of drugs to have been created; the three scientists credited for the discovery of monoclonals won the Nobel Prize for Medicine in 1984.
SAb Biotherapeutics also had a strong Iowa connection for many years. Trans Ova Genetics, in Sioux Center, Iowa, is the largest bovine embryo transfer company in the world. SAb and its predecessor companies contracted with Trans Ova for embryo transfer and management of its bovine herd for about 15 years, before taking this work over itself.
The Long Road from Anthrax Poisoning to Type 1 Diabetes
Hematech took the first steps toward making its fully human polyclonal pharmaceuticals via cloned bovines in 2001, following the anthrax poisoning scare in the United States. Several top elected officeholders and other people of note began receiving letters laced with anthrax powder. There was no cure for anthrax poisoning and there were several deaths. Hematech and several other pharmaceutical companies were asked by the federal government to try to find a cure for anthrax poisoning.
The work progressed a bit, but before the lengthy process of making a new drug, testing it, and getting it approved for use by the Federal Drug Administration could be completed, the anthrax letters ceased arriving. The work on creating a cure for anthrax poisoning ceased because it would have to be tested eventually on human patients, and there were no anthrax poisoning cases in America anymore.
Over the next 20 years or so, work was begun in the development of cures for various infectious diseases that looked like they might reach pandemic level – and one that did reach that level. Much of the work was financed by the federal government. Ebola, Hantavirus, Zika, MERS, and finally COVID-19 all became the focus of federally financed fully human polyclonal antibody development at SAb and its predecessor companies. Each time, the polyclonal therapeutics looked like they were going to work, and a few times, clinical human trials were begun. Every one of these diseases, however, gradually decreased as a world threat before testing could be completed.
SAb did find one desperate patient with a rare life-threatening disease in 2016, which provided an unusual chance to test one of its therapeutics on a real person in a real-world situation. Special arrangements were made for SAb to develop a therapeutic specifically for this patient’s rare disease – an infection that prevented the healing of a surgical wound from a hip transplant. The wound would not close and became endlessly infected. It would not respond to any known antibiotic. SAb agreed to develop a fully human polyclonal antibody from a cloned bovine specifically for this patient. The man, in Boston, received the SAb therapeutic made for him. Miraculously, after having an open infected wound for seven years, the wound closed and healed. Sadly, however, the man died a few years later from other complications of his illness.
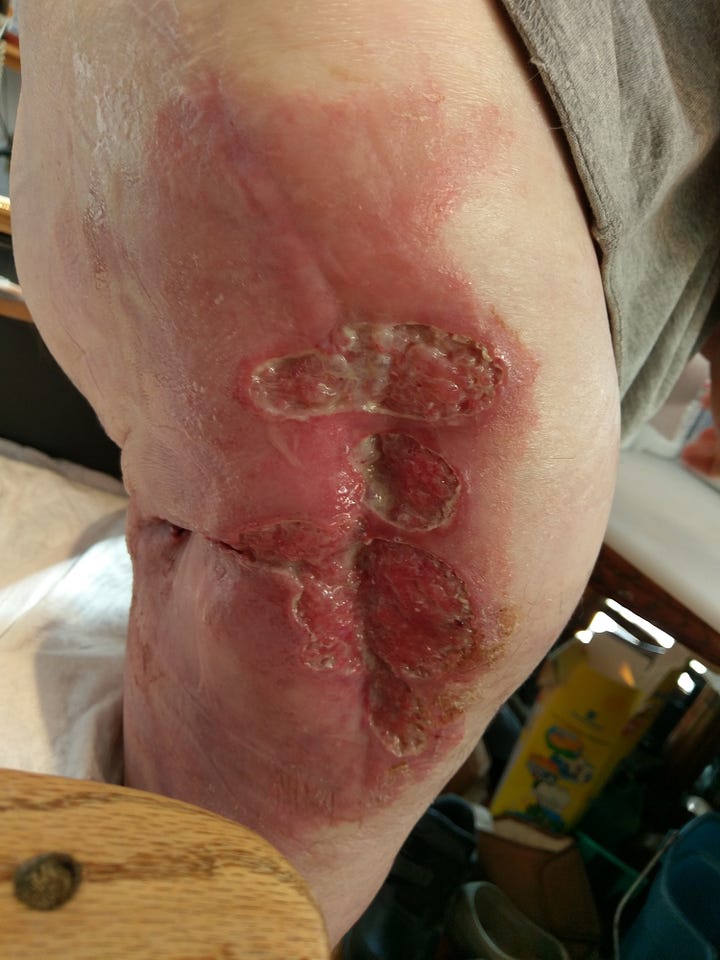

In recent years, SAb has shifted gears and begun developing, manufacturing, and testing fully human polyclonal therapeutics from cloned bovines for diseases that are more common and have few options for cure.
It is an expensive process. Each specific therapeutic can cost hundreds of millions of dollars to take through the FDA’s clinical testing process and require several years to be approved for human use.
SAb’s primary area of focus today is developing its therapeutic for Type 1 diabetes and getting it to market. The therapeutic shows promise for doing something that no drug has been able to do: Delaying the onset or slowing the progression of Type 1 diabetes with no more than an annual treatment. Human trials began recently in Australia.
In North America, 38 percent of the total deaths of people under the age of 60 are a result of Type 1 diabetes (T1D). In Europe, T1D accounts for 50 percent of the deaths under age 60; in Africa, 76 percent. In the U.S. alone, about 250,000 children and 1.5 million people total are living with T1D.
Influenza and C. diff. therapeutics also are in development. The worldwide death toll for influenza ranges from 300,000 to 500,000 each year. For C. diff., the Center for Disease Control estimates 500,000 cases and 30,000 deaths in the U.S. each year.
Work also has been in an early stage on a therapeutic for non-small-cell lung cancer. There are about 200,000 new cases and 130,000 deaths from this type of cancer in the U.S. every year.
The Final Big Step: Money, Money, Money
The cost of clinical testing for these therapeutics has been the biggest hurdle in SAb’s efforts to get its therapeutics into the marketplace. As a startup company with no therapeutics yet approved for use and a very modest revenue stream, it has had a difficult time raising money.
Within the past year, however, there has been progress in this area. A coalition of venture capital companies recently committed $130 million for clinical testing that could go a long way in helping to bring the Type 1 diabetes therapeutic to market. Also, the managing director of the Juvenile Diabetes Research Foundation T1D Fund, which has $200 million in assets, including an investment in SAb, has joined the SAb board.
Finally, in terms of hope for the future, consider this: Among all the pharmaceutical companies in the world, the failure rate for new drugs in clinical testing is 90 percent. The failure rate thus far for SAb’s polyclonal therapeutics has been: Zero.







The book was privately published and is not available on the public market. I may be able to produce a few copies myself in a spiral bound format. I will be happy to provide copies at my cost to produce, which is $50. Note that it may be a few weeks before the clients are ready to distribute their copies, and I don't want to do anything until that happens. From your note, I think the book may strike a lot of chords with you. It begins with Von Behring and Ehrlich and travels through penicillin and monoclonals before arriving at Hematech and SAb and what is essentially a new class of therapeutics. The story of Balto is included as well. I will keep you updated on this. It might be easier to convert to email for this continuing discussion. I'll get that started.
Very interesting read, a welcome respite from caucus crap. Targeted therapy is the future for so many conditions. What an interesting writing project for you Arnie. Hope your treasured lake place was not near the fire.